What is SPECTA?
SPECTA is a collaborative European platform to reach patients outside of clinical trials and establish a quality-assured process for collecting clinically and pathologically annotated biological material from cancer patients. The goal of such a platform is to support bio specimen-based translational research, biomarker discovery and ultimately to propose new therapeutic options to patients with cancer.
The platform provides an integrated and shared mechanism, with rapid access to patient data and biological samples to enable the quick implementation of new clinical trials and robust translational research.
What is SPECTA?
SPECTA is a collaborative European platform to reach patients outside of clinical trials and establish a quality-assured process for collecting clinically and pathologically annotated biological material from cancer patients. The goal of such a platform is to support bio specimen-based translational research, biomarker discovery and ultimately to propose new therapeutic options to patients with cancer.
The platform provides an integrated and shared mechanism, with rapid access to patient data and biological samples to enable the quick implementation of new clinical trials and robust translational research.
How does SPECTA work?
Platform Status
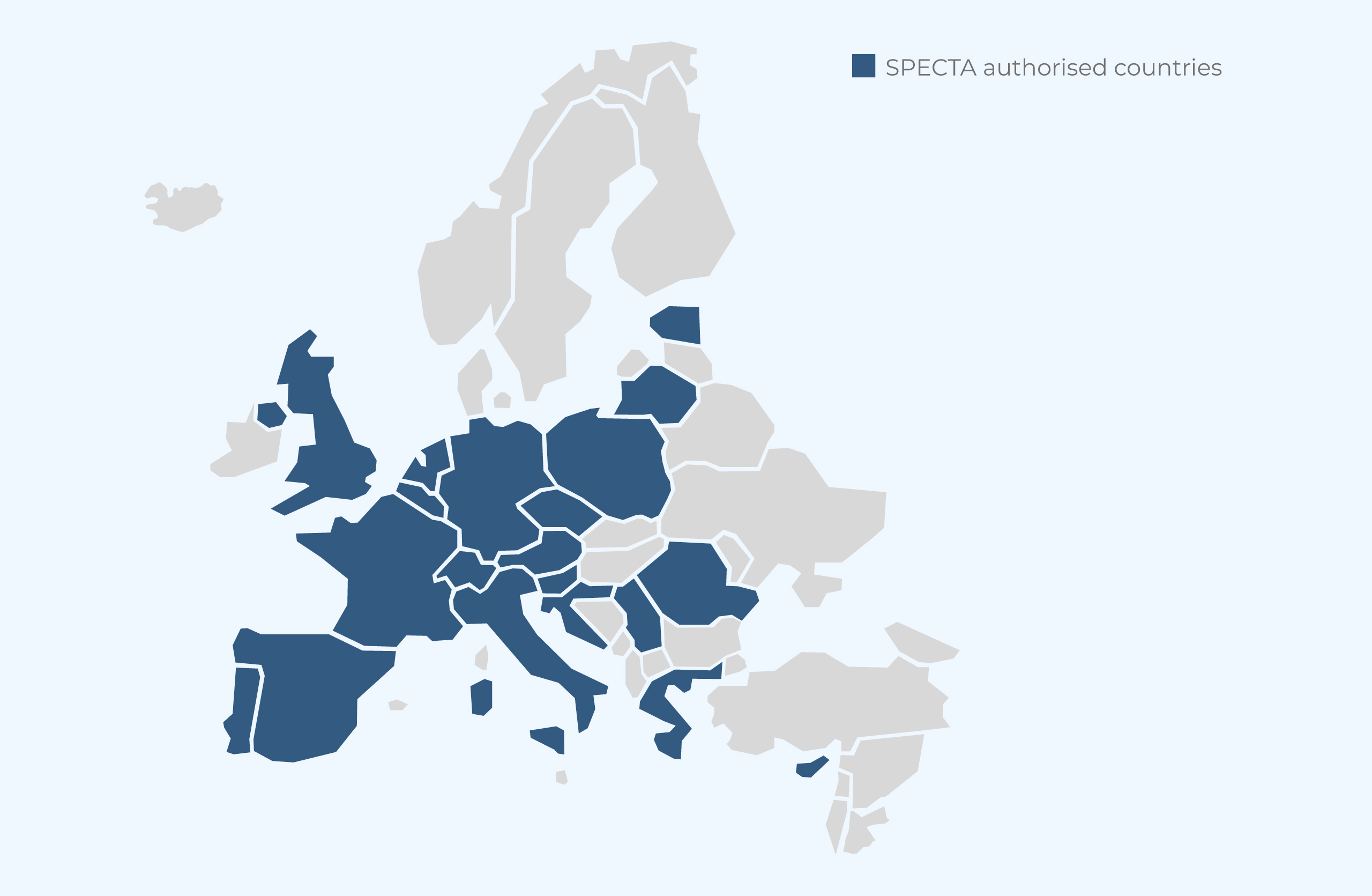
SPECTA is authorising new investigators on an ongoing basis. We are proud of our SPECTA network, active all over Europe.
Since the initiation of the SPECTA platform and its first downstream projects mid-2019, approximately 600 cancer patients annually have been confirmed as eligible for participation.
SPECTA in numbers (March 2024):
- 168 investigators authorised to recruit
- 20 countries represented
- Around 3870 patients registered
- 2790 patients eligible for a downstream project
- More than 2000 molecular reports generated
- 10 cohorts opened to recruitment
- 11 cohorts under analysis after reaching targeted recruitment
- 5 downstream projects: 2 projects recruiting, 1 in analysis, 1 in activation
- A team of 25 people at EORTC headquarter dedicated and involved either in the platform or its downstream projects.
Find out more about the current open sites:
Sponsorship
The SPECTA platform is supported by Alliance Healthcare.
Alliance Healthcare will become Cencora.